the particulate nature of matter
Names of changes of states

| Change of state | Description | Exothermic or Endothermic? |
| Melting | Solid to Liquid | Endothermic (absorbs energy) |
| Vaporisation ( Boiling or Evaporation) | Liquid to gas |
| Sublimation | Solid to Gas (No inbetween state) |
| Condensation | Gas to liquid | Exothermic (releases energy) |
| Freezing | Liquid to solid |
| Deposition | Gas to solid ( No inbetween state) |
Elements, Compounds, molecules
Elements: Single substances composed of atoms of the same type.
Compounds: Substances composed of two or mole different elements bonded together. They may have different properties to the original.
Molecules: Two or more atoms bonded together.
Ratio of elements in a compound
Find the ratio of elements in a compound by balancing the charges. Do this using the periodic table.
E.g Mg has charge +2 and Cl has charge -1, so the compound needs one Mg to two Cl to balance the charges. MgCl2
Mixtures
These contain more than one element or compound which are not chemically combined, so they retain their individual properties.
Homogeneous: The mixture is mixed such that the elements/compounds are distributed equally throughout.
Heterogeneous: The mixture is not uniform in composition
Deducing chemical equations from reactants and products
Chemical equations summarise the change between reactants and products.
To deduce the chemical equation:
- Use the data booklet to convert the reactants and products to their corresponding symbols
- Set up the equation with the reaction arrow and symbols.
- Apply correct state symbols (s), (l), (g) and (aq)
- Equate coefficients so that the same number of elements are either side.
Note: a compound is aqueous if it is soluble in water. Remember like dissolves like.
The mole concept
Relative atomic and molar mass
Relative Atomic mass (Ar): The mass of an element relative to carbon-12, where Carbon-12 is defined to have a mass of 12.
Molar mass (Mr) : This is calculated by summing the atomic masses of each element in a compound. The unit for molar mass is g mol-1
What are moles
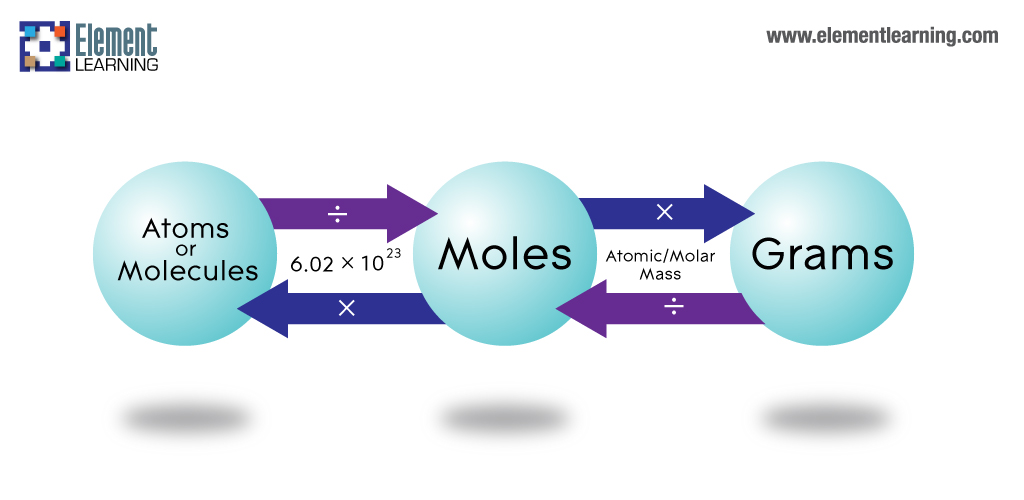
Moles are used to count particles because particles are so unbelievably small that a huge number of them can fit in a beaker. One mole of a something is simply 6.02 x1023 times of it. It has the symbol n.
Avogadro's number: The number of particles which equal one mole - 6.02 x 1023
Converting between particles,moles and grams
- To convert from moles to particles multiply the number of moles by avogadro's number
- To convert from moles to grams multiply the number of moles by the molar mass(Mr)
- When going the other way divide instead of multiplying
Example question 1
What is 0.2g of Al2O3 in moles?
- Molar mass (Mr) of Al2O3= 27*2+16*3 = 102g/mol
- Moles = 0.2/102 = 0.002 mol
Example question 2
A sample of magnesium contains 1.41 * 10^21, calculate the number of moles
Mole ratio
The mole ratio is the ratio between the moles of different compounds in a chemical formula.
You can find the mole ratio by looking at the coefficients in front of the compounds in the formula.
Example:
- The mole ratio between the oxygen and water is 1:2, as for every one mol of oxygen reacted, 2 moles of water is produced.
- It is a 1:1 mole ratio between Hydrogen and water, as they have the same coefficients.
Empirical and molecular formula
Empirical formula: This is the simplest whole number ratio of elements in a compound. It is important that the ratio is in its simplest form. If the ratio can be simplified it must. E.g H4O2 is not an empirical formula because it can be simplified to H2O
Molecular formula: This tells the number of moles of each element. It is the same as the empirical formula multiplied by some whole number.
Finding the empirical formula given percentage composition
- Assume that the compound has a mass of 100g.
- Calculate the mass of each element by multiplying the percentage by 100g
- Divide each mass by the atomic mass to find the moles of each element.
- The simplest integer ratio between the moles of element is also the ratio in the empirical formula
Find the Molecular formula given mass and the empirical formula
- With the empirical formula find the molar mass of the compound, with the simplified ratio.
- Divide the mass given by the molar mass of the empirical formula, to get a whole number.
- Multiply the empirical formula by this whole number to get the molecular formula.
Reacting Masses and volumes
Limiting and excess reagents
Limiting reactant: This is the reactant which determines the theoretical yield.
Excess reactants: All reactants which aren't the limiting reactant are in excess, so they don't affect the yield.
Determine the limiting reactant
If given the chemical equation and the masses do this:
- convert the masses to moles
- Divide the moles by the mole ratio coefficients
- The reactant with the smaller number is the limiting reactant
Experimental and theoretical yield
The yield gained in a experiment is often lower in an experiment due to errors with the method. The percentage yield can tell us how much of the limiting reactant actually reacted.
Theoretical yield: The yield expected theoretically, assuming all the limiting reactant is converted into products.
Experimental yield: The yield in an experiment
Percentage yield:
Ideal gas
This is a gas that exhibits the five postulates of the kinetic molecular theory, as well as obeying the gas laws.
Properties of an ideal gas
- The particles in a gas exhibit random motion
- There are no intermolecular force between particles
- All collisions are perfectly elastic
- Particles have negligible volume
- The kinetic energy is directly proportional to temperature
Gas laws
Combined gas law:
Ideal gas equation: PV = nRT
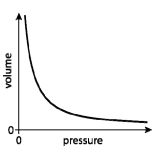
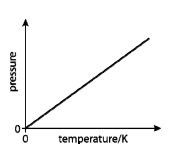
Note: By rearranging the ideal gas equation you can find the relationship between any two components
Avogadro's Law
Avogadro’s Law states that equal volumes of gases at the same temperature and pressure contain equal numbers of particles. This is true because a gas is mostly empty space so the the nature of the particles themselves does not affect the number of particles which are in a gas. This law is important because it means any same volume of gas also has the same number of molecules and hence the same number of moles.
Molar volume: 22.7 dm3 mol-1 - This is the amount of volume one mole of idea gas takes up under STP
Solutions
Concentration = mol dm-3
1 dm3 = 1L
Making a standard solution
- Weigh out a specific mass of solute with a high precision scale
- Dissolve the mass in distilled water, and pour the solution in a volumetric flask.
- Make up the volumetric flask to the line with more distilled water.
Volumetric analysis
List of apparatus
| Apparatus | Description | Diagram |
| Beaker | A container for liquids with a large volume and open top. (Should not be used for precise measurements) |  |
| Conical flask | A container used in titrations. they are tapered at the top to allow solutions to be swirled without the risk of spillage. |  |
| Measuring cylinder | A cylinder with precise measurements along the side. Used to measure volumes. |  |
| Volumetric flask | They can measure specific volumes very precisely. Used to create standard solutions. They have a line on their neck indicating where to fill the liquid up to. |  |
| Burette | Used to deliver a precise volume of a solution during a titration. |  |
Acid-Base Titration
A Titration is a method of finding the concentration of an unknown solution using a solution with a known concentration.
Titrant: The solution with the known concentration in buret, can be an acid or a base
Analyte: The solution with the unknown concentration in conical flask, can be an acid or a base.
Procedure:
- Rinse out burette and fill conical flask with a Standard solution of analyte. Add an indicator like Phenolphthalein
- Perform test trial to get a rough idea how much titrant is needed to change the colour.
- Slowly let out titrant into the conical flask, until the colour changes indicating the analyte has been neutralised.
- Record the volume of titrant needed then repeat three times to get an average.
Concentration Titrant =
View count: 18777